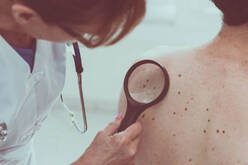
Novel nanoplatforms for targeting melanoma with 8-hydroxyquinoline metal complexes
Cancer is one of the most challenging diseases and cancer-related deaths are estimated to rise as life expectancy increases. Melanoma is the sixth most frequently diagnosed cancer and the most common malignant skin neoplasm. Once metastasized, it usually becomes fatal due to the scarce therapeutic options available. In this project, we propose to develop new metallodrugs based on a bioactive scaffold, 8-hydroxyquinoline (8HQ), aiming at stable, efficient and selective compounds. The metal ions selected are Cu(II), V(IV) and Zn(II), as these also have documented therapeutic potential. Following the initial characterization, the compounds will be screened in vitro in different human and murine melanoma cells, and the most active will be selected for encapsulation in liposomes and polymeric nanoparticles, since their association to drug delivery systems is a valuable strategy that may provide improved efficacy and selective drug targeting. The evaluation of the cellular uptake of the compounds as well as the study of the mechanisms of cell death will be carried out with the most promising compounds identified in the cellular screening. Compounds selected in in vitro screenings will be tested in vivo in immunocompetent murine melanoma models.
The strategy encompasses aggregating distinct approaches so that the combined results will guide the design towards compounds with optimal performance. This ambitious project faces numerous challenges, however the team is multidisciplinary and highly skilled with the required expertise. The researchers have already successfully collaborated and preliminary data supports the proposed strategy to obtain active drugs for melanoma therapy. We, thus, predict that the use of structure-activity relationships will enable the selection of lead compounds for pre-clinical trials.
This project was funded by FCT (PTDC/QUI-QIN/0586/2020)
Find project updates in the project website.

Reactivity of transition metal complexes in ionic liquids
The reaction rate is completely inseparable from the medium in which it is performed. Solvents influence the outcome of reactions because they interact differently with solutes. Therefore, the chemistry conducted in ionic liquids (ILs) is totally different from chemistry in the environment of a molecular solvent. This project aims to study how ILs interact with transition metal complexes and to determine how that interaction changes the complex reactivity.
An important field in which ILs and oxidation catalysts are being applied is the desulfurization of fuels. To achieve deep desulfurization oxidation reactions are a promising procedure and the combination with ILs extraction is a state of the art application. This background prompted us to explore the catalytic potential of the vanadium complexes for the oxidation of dialkyldibenzothiophenes in molecular solvents and ILs. The oxidative desulfurization is claimed to be cost-effective compared to the hydrodesulfurization treatment process because it can be performed in ambient temperature without using hydrogen. Furthermore, the most attractive intrinsic aspect is the anticipated higher reactivity of more aromatic sulfur species, which are very difficult to remove by the hydro process. Extraction experiments with different ILs and mixtures will provide results that will access the potentialof the oxidation /extraction system for the production of ultra low sulfur fuels.
This project was funded by FCT (PTDC/Qui-Qui/098516/2008)
The reaction rate is completely inseparable from the medium in which it is performed. Solvents influence the outcome of reactions because they interact differently with solutes. Therefore, the chemistry conducted in ionic liquids (ILs) is totally different from chemistry in the environment of a molecular solvent. This project aims to study how ILs interact with transition metal complexes and to determine how that interaction changes the complex reactivity.
An important field in which ILs and oxidation catalysts are being applied is the desulfurization of fuels. To achieve deep desulfurization oxidation reactions are a promising procedure and the combination with ILs extraction is a state of the art application. This background prompted us to explore the catalytic potential of the vanadium complexes for the oxidation of dialkyldibenzothiophenes in molecular solvents and ILs. The oxidative desulfurization is claimed to be cost-effective compared to the hydrodesulfurization treatment process because it can be performed in ambient temperature without using hydrogen. Furthermore, the most attractive intrinsic aspect is the anticipated higher reactivity of more aromatic sulfur species, which are very difficult to remove by the hydro process. Extraction experiments with different ILs and mixtures will provide results that will access the potentialof the oxidation /extraction system for the production of ultra low sulfur fuels.
This project was funded by FCT (PTDC/Qui-Qui/098516/2008)
Synthesis, Structure and Reactivity of Transition Metal Complexes with Potential Application in Oxidative Catalysis
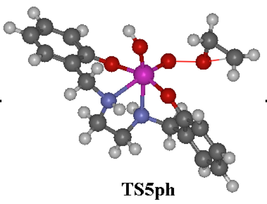
The fine chemicals industry still abounds with classical technologies that generate large amounts of inorganic waste. However, it is under strong pressure to reduce its environmental impact. Much attention has been focused on the application of transition metal catalysts to promote organic transformations. The demand for functionalized materials of high optical purity has led to an intense research effort in asymmetric catalysis.
The goal of this project was to contribute to the development of environmental-friendly processes, which will lead to the implementation of Green Chemistry concepts. The aim was to design, synthesize and test new and efficient transition metal catalysts for the enantioselective synthesis of chiral sulfoxides and epoxides, which will allow the use of greener processes.
This project was funded by FCT (POCI/QUI/55985/2004)
The goal of this project was to contribute to the development of environmental-friendly processes, which will lead to the implementation of Green Chemistry concepts. The aim was to design, synthesize and test new and efficient transition metal catalysts for the enantioselective synthesis of chiral sulfoxides and epoxides, which will allow the use of greener processes.
This project was funded by FCT (POCI/QUI/55985/2004)
